110 faulty bottles of Sinovac vaccine rejected by Thai FDA
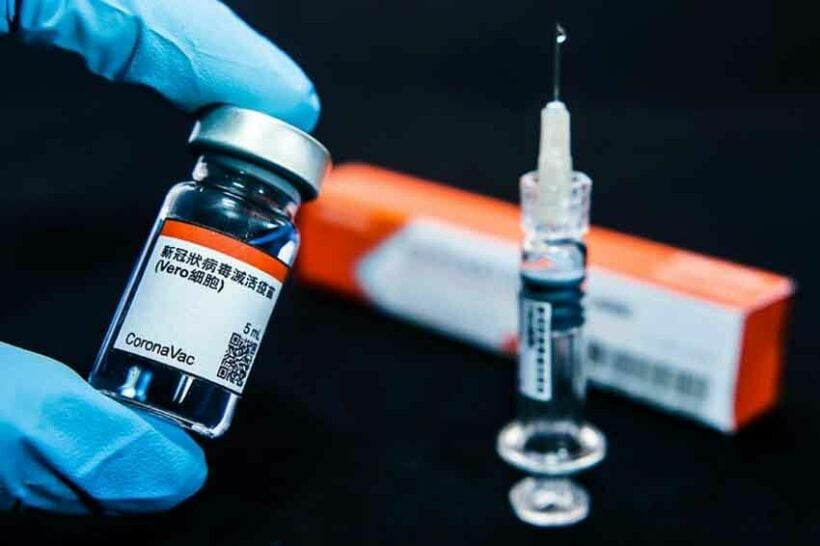
The Food and Drug Administration says it has found 110 faulty bottles of the CoronaVac vaccine from Sinovac Biotech that contain lumps of gel. Surachok Tangwiwat from the FDA says CoronaVac vials need to be shaken prior to use but, in the case of the defective bottles, even shaking would not dissolve the gel.
According to a Coconuts report, the FDA has issued a statement confirming that 110 bottles have been found to be defective, instructing healthcare workers not to administer them. The C202105079 batch was manufactured on May 19 and has an expiry date of November 9. The doses were produced in China and shipped to Thailand, with improper storage and transportation being blamed for the defects. Sinovac needs to be kept at temperatures of between 2° and 8° Celsius.
Over 10 million doses of Sinovac have arrived in the Kingdom from China, with millions more expected this year. The Public Health Ministry says the vaccine has been found to be between 71% and 91% effective against the Alpha variant of the virus. However, doubt has been cast on its efficacy against the highly-contagious Delta variant first reported in India.
SOURCE: Coconuts
Latest Thailand News
Follow The Thaiger on Google News: