3 Covid-19 vaccines approved by Thai FDA, 3 more in registration process

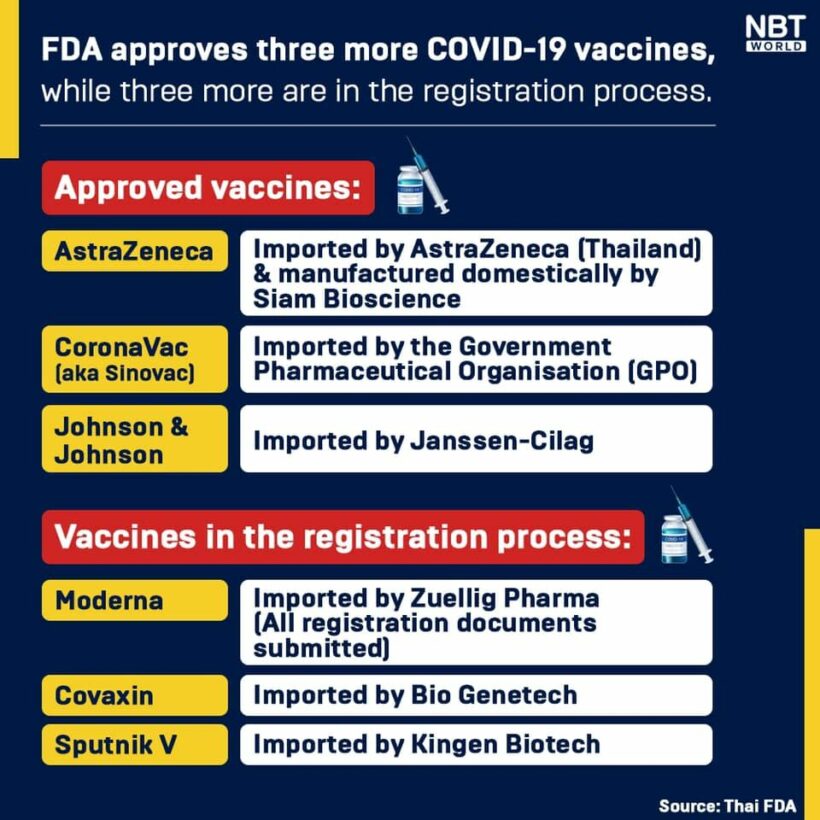
3 Covid-19 vaccines have been approved by the Thai Food and Drug Administration while 3 more pharmaceutical and biotech companies are in the process of registering Covid-19 vaccines for use in Thailand, according to FDA secretary general Phaisan Dankhum.
So far, the FDA has approved the AstraZeneca Covid-19 vaccine, which was made in partnership with Oxford University in the UK and now being produced locally by Siam Bioscience. The FDA also approved the Johnson & Johnson Covid-19 vaccine and China’s CoronaVac vaccine, which was produced by Sinovac.
Moderna has submitted all of the required documents to the Thai FDA and Phaisan says it should be registered for use in Thailand this month. He says the administration is currently receiving registration documents from Biogenetech for its Covaxin vaccine and KinGen Bioteach for its Sputnik V vaccine.
The Thai government has also shown interest in the Pfizer vaccine, which studies show is safe for children 12 and up. However, no steps have been made in the registration process.
PM Prayut Chan-o-cha set a goal to vaccinate more than half the population by the end of the year. It’s a little unclear if expats are included in that goal.
In a press briefing last week, a spokesperson from the Centre for Covid-19 Situation Administration said expats in Thailand will be included in the national rollout of Covid-19 vaccinations and an immunisation plan for foreigners would be released at a later date. On the other hand, a spokesperson from the Public Health Ministry recently said that Thais will have priority in the next stage of vaccinations due to the limited number of doses, adding that expats will need to wait until Thailand has a surplus of vaccines.
“The vaccines right now are only reserved for Thai people who are now at a high-risk level or living in the severe outbreak areas.”
Latest Thailand News
Follow The Thaiger on Google News: