Thailand begins landmark dengue vaccine trial for children
Thailand is taking a major step in the fight against dengue fever, launching a large-scale clinical trial of a new vaccine for children in Nakhon Phanom province. The trial, set to begin on April 4, will involve 35,000 children aged between seven and 10, regardless of prior dengue infection.
Approved by the National Communicable Disease Committee, the study aims to determine the vaccine’s effectiveness and pave the way for its inclusion in Thailand’s universal healthcare scheme.
“The testing should be completed in three years, after which the vaccine can be included in the country’s universal healthcare scheme,” said Dr Panumas Yanawetsakul, Director General of the Department of Disease Control (DDC).
The vaccine, developed by a Japanese pharmaceutical company and registered with the Thai Food and Drug Administration (FDA), is a live-attenuated type, similar to the measles vaccine.
Dr Nakorn Premsri, director of the National Vaccine Institute, explained that while the trial is promising, adding the vaccine to the national healthcare programme would take time.
“But adding the vaccine to the universal healthcare scheme will take time, pending the approval of the subcommittee on immunisation after reviewing the test results versus cost-effectiveness.”
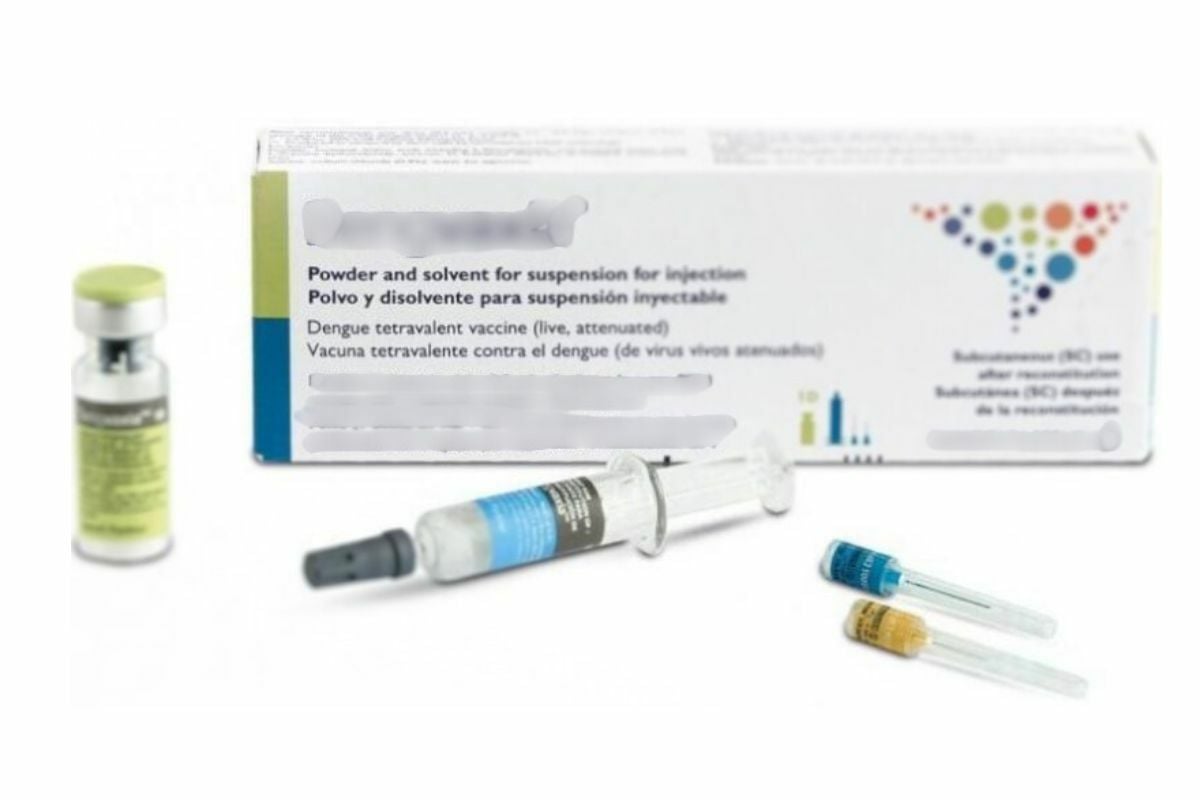
Currently, dengue vaccines are available in Thailand but they remain an expensive alternative offered mostly by private healthcare providers. The government’s move to introduce a publicly funded vaccine could make dengue immunisation widely accessible and affordable for millions.
Dengue fever, a mosquito-borne disease, is endemic in over 100 countries and poses a significant health risk in tropical regions like Thailand. The vaccine trial represents a crucial effort to curb infections and reduce the burden on the healthcare system.
With Thailand reporting thousands of dengue cases annually, health experts hope that a successful trial will lead to mass immunisation, potentially saving countless lives.
The government remains committed to closely monitoring the trial’s progress and ensuring that, if successful, the vaccine will be integrated into public healthcare as soon as possible, reported The Nation.
Latest Thailand News
Follow The Thaiger on Google News:


























