New rapid Covid-19 tests await FDA approval
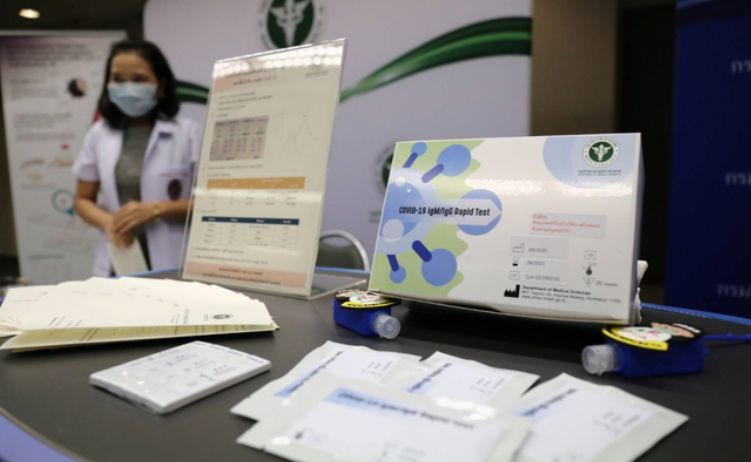
A new rapid Covid-19 test made in Thailand is expected to cut coronavirus screening costs and provide results within a day. The tests, developed by the Medical Sciences Department in collaboration with Siam Bioscience, are waiting to be approved by the Food and Drug Administration. The approval process could take around a month.
The test, once proven and rolled out, could provide another path to shortening, or eliminating, the quarantine period, currently set at 14 days in Thailand.
The antibody rapid tests read serum samples taken from plasma and blood in fingertips, according to the department’s acting director general. It’s more than 90% accurate. The tests could cost around 100 baht each. At the moment, the department has the capacity to produce 3,000 tests per week, but says they are ready to transfer technology to commercial producers to make more tests.
Thailand has 236 Covid-19 laboratories that check more than 20,000 samples per day, with 10,000 of those samples in Bangkok, according to Nation Thailand.
Once the test is approved and registered by the FDA, Nation Thailand says the test will be used in a random screening of 60,000 people to review the prevalence of Covid-19 in Thailand.
SOURCE: Nation Thailand
Latest Thailand News
Follow The Thaiger on Google News: