Death of 66 children linked to an Indian cough medicine

The World Health Organization (WHO) believe the death of 66 children in The Gambia could be linked to a cough medicine made in India.
The Gambia’s director of health services, Mustapha Bittaye, last month revealed that dozens of children under the age of five died in the past three months from kidney failure that may have been caused by a paracetamol syrup.
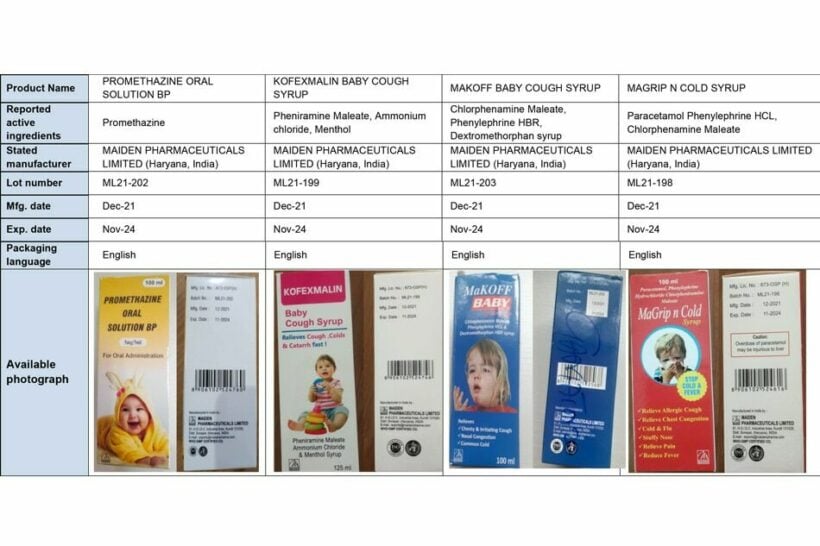
The medicines, manufactured by an Indian company, Maiden Pharmaceuticals, have been identified as Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
The WHO alleges the Indian company failed to provide guarantees about the medicines’ safety.
WHO Director-General Tedros Adhanom Ghebreyesus reported that an investigation is underway.
The Gambia’s government, however, has suspended the use of all paracetamol syrups and urged people to use tablets instead.
A WHO laboratory products test revealed that the medicines “contain unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.”
The substances are toxic and they “can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death.”
The WHO added that all batches of these products should be considered unsafe until they can be analyzed by the relevant National Regulatory Authorities.
Ghebreyesus said…
“The loss of these young lives is beyond heart-breaking for their families.”
The Gambia medical officers brought it to public attention in July after a number of children began suffering from kidney problems three to five days after taking the cough medicine. Twenty-eight children had died by August 28. Now 66 are reported dead.
Maiden Pharmaceuticals manufactures medicines at its facilities in India. They sell the cough medicine at home and also export it to Asia, Africa, and Latin America.
Maiden Pharmaceuticals, The Gambia health ministry, and India’s health ministry have not provided any comments as yet.
Latest Thailand News
Follow The Thaiger on Google News: