It’s a yes: WHO approves Sinovac for emergency use
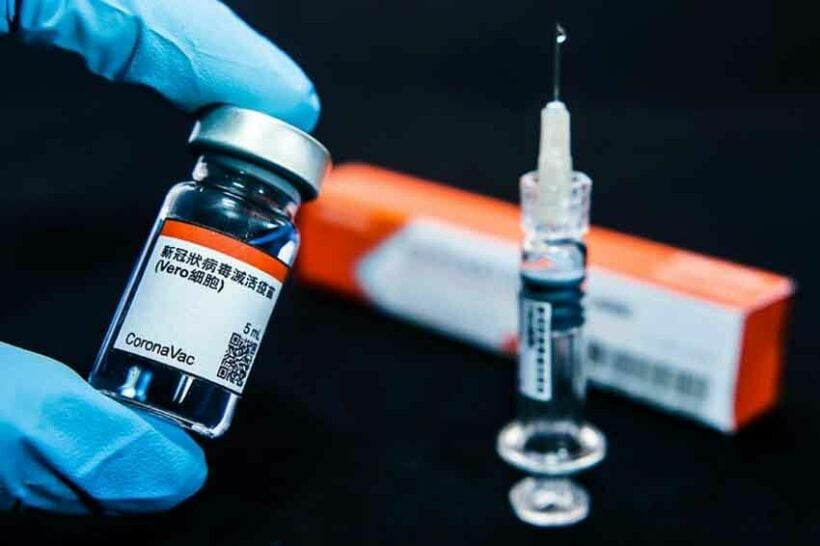
The CoronaVac vaccine from Chinese firm Sinovac has been approved for emergency use, making it the second Chinese vaccine to be approved by the World Health Organisation. The approval paves the way for more widespread global distribution, particularly as part of the Covax scheme. The scheme aims to provide poorer countries with equal access to Covid-19 vaccines but up to now, only AstraZeneca and Pfizer doses could be distributed.
According to a Bangkok Post report, WHO boss Tedros Adhanom Ghebreyesus confirmed the approval of Sinovac at a press conference, adding that the vaccine has passed all safety and efficacy checks.
“I’m happy to announce that the Sinovac-CoronaVac vaccine has been given WHO emergency use listing after being found to be safe, effective, and quality-assured. The easy storage requirements of CoronaVac make it very suitable for low-resource settings. It’s now crucial to get these life-saving tools to the people that need them quickly.”
The endorsement follows the WHO’s approval last month of another Chinese vaccine, Sinopharm, which the Chulabhorn Royal Academy plans to import to Thailand to supplement the national rollout here.
The WHO says Sinovac’s approval can reassure the world that the vaccine has met with international standards. Other vaccines previously approved for emergency use by the WHO include Pfizer, Moderna, Johnson & Johnson, and the AstraZeneca vaccine being manufactured in India, South Korea, and the EU.
The WHO says Sinovac’s approval means countries around the world, particularly those without their own system of regulatory approval, can now feel confident in approving and importing the vaccine.
“The world desperately needs multiple Covid-19 vaccines to address the huge access inequity across the globe. We urge manufacturers to participate in the Covax facility, share their know-how and data and contribute to bringing the pandemic under control. WHO recommends the vaccine for use in adults 18 years and older, in a 2-dose schedule with a spacing of 2 to 4 weeks.”
According to studies, Sinovac prevents symptomatic infection in 51% of vaccinated people and prevents severe illness and hospitalisation in 100% of those vaccinated. While few people over the age of 60 participated in clinical trials, the WHO says there’s no reason to believe it’s less safe in the older age group.
SOURCE: Bangkok Post
Latest Thailand News
Follow The Thaiger on Google News: