Sinovac and AstraZeneca: The 2 primary Covid-19 vaccines in Thailand
Since Thailand started its mass Covid-19 immunisation campaign in late February, the Chinese-made Sinovac vaccine and the AstraZeneca vaccine, made in partnership with the UK’s Oxford University, have been used to vaccinate residents in Thailand.
The local firm Siam Bioscience is now producing the AstraZeneca vaccine and the first Thai-made batch is expected to be rolled out next month.
Type of vaccine
AstraZeneca: A recombinant vaccine from a modified chimpanzee adenovirus.
This is a harmless, weakened adenovirus that usually causes the common cold in chimpanzees. The adenovirus vaccine vector, known as ChAdOx1, was chosen as a suitable vaccine technology for a SARS-CoV-2 vaccine as it has been shown to generate a strong immune response from one dose in other vaccines. It has been genetically changed so that it is impossible for it to grow in humans. – Australian Department of Health
Sinovac: An “inactivated” vaccine, using inactivated virus particles to produce an immune response, a traditional approach for vaccines and the same technology used to produce the flu and polio vaccines.
The World Health Organisation says the vaccine is made by inactivating or killing the virus using chemicals, heat or radiation.
Effectiveness
Studies are still underway for both vaccines, while more studies of the AstraZeneca vaccine have been completed and reported. In Thailand, a recent study by Chulalongkorn’s Centre for Excellence in Clinical Virology of the Faculty of Medicine found both vaccines effective after both doses.
According to the university study…
- 99.49% of Sinovac recipients developed antibody reponses 4 weeks after their second injection.
- 97.26% of AstraZeneca recipients developed antibody responses 4 weeks after their first injection.
Thailand initially used the Sinovac vaccine for people ages 18 to 59 due to limited research for the 60 and up age group. Thai health officials recently announced that recent studies show the Sinovac is safe and effective for adults over 60 years old who are in good health.
Global travel
A dilemma for many expats living overseas, or those wanting to travel in the future, is which Covid-19 vaccine to get as some are not recoginsed by other countries.
Both Sinovac and AstraZeneca have met the World Health Organisation’s criteria for safety and efficacy. The Sinovac vaccine is awaiting final approval.
Some countries may not recognise certain vaccine passports, although international tourism is still in the early stages and proposed regulations are constantly changing.
Thailand recoginses vaccines that are either approved by the World Health Organisation or by the Thai government.
The European Union plans to reopen tourism to American travellers, but they may need to have a vaccine approved by the European Medicines Agency.
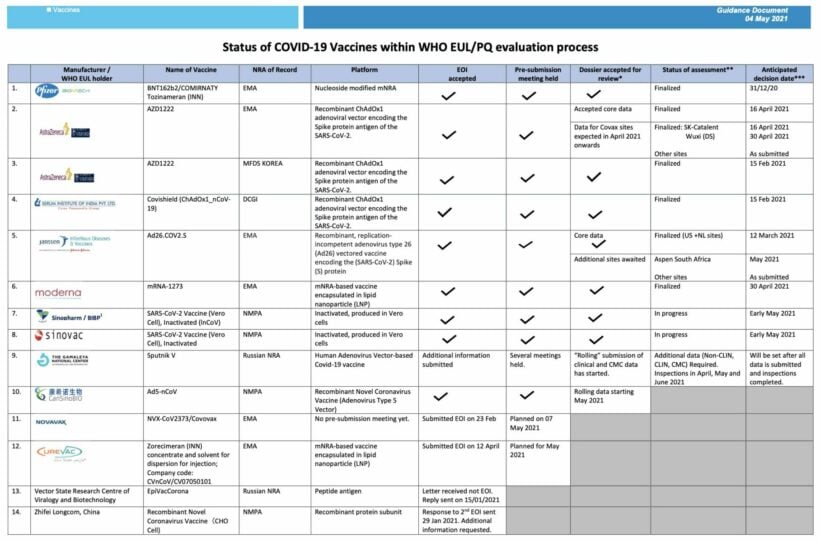
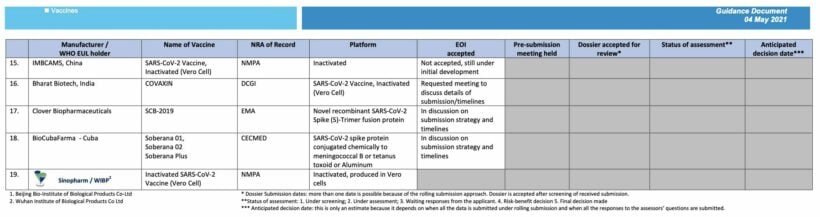 SOURCES: WHO | Healthline
SOURCES: WHO | Healthline
Latest Thailand News
Follow The Thaiger on Google News:


























