Thailand launches first local cancer therapy pill
Princess Chulabhorn oversaw inspections and research in the lab personally
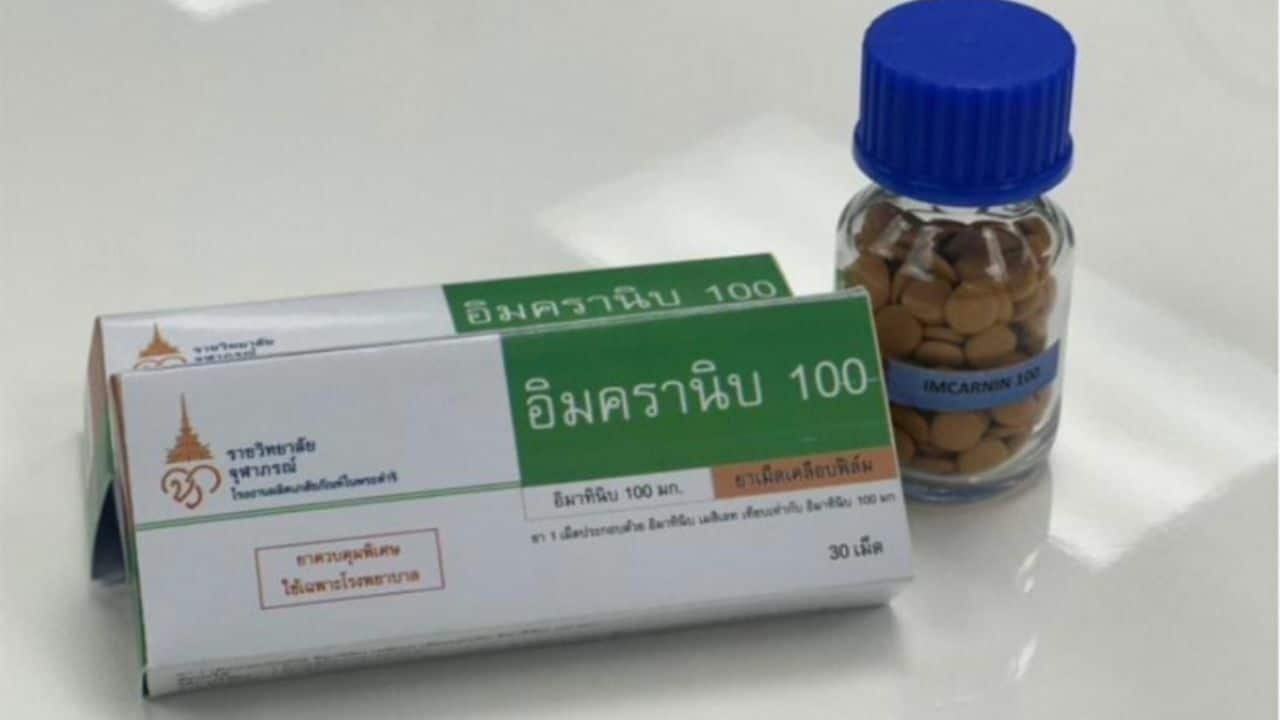
Thailand has introduced its first domestically created targeted therapy pill for cancer treatment, named Imcranib 100. This development is attributed to the scientific vision and leadership of Her Royal Highness Princess Chulabhorn Krom Phra Srisavangavadhana.
Targeted therapy represents a modern approach to cancer treatment, concentrating on attacking specific cancer cells while preserving healthy cells. This method helps to minimise the side effects typically associated with traditional treatments like chemotherapy, as noted by the Chulabhorn Royal Academy (CRA).
According to the CRA, this approach also facilitates the creation of more personalised treatment plans that are tailored to each patient’s unique condition.

Motivated by the needs of cancer patients with limited access to effective medicines, Princess Chulabhorn established a pharmaceutical manufacturing facility under royal initiative at Phimanmas Residence in Sattahip district, Chon Buri, in 2020.
The facility, operating under the CRA, is Thailand’s inaugural cancer drug manufacturing site certified to global GMDP PIC/s standards. Its creation was intended to secure domestic production capabilities from research through to industrial scale, aiming to decrease reliance on expensive drug imports and to enhance national pharmaceutical expertise.
ดูโพสต์นี้บน Instagram
Princess Chulabhorn personally monitored the plant’s progress, engaging in inspections and scientific operations within the quality control laboratory. She spearheaded efforts to uphold international standards in drug development, conducting chemical and physical analyses of raw materials and finished products.

The culmination of these initiatives is Imcranib 100, a tablet containing 100 milligrammes of Imatinib, which was registered with the Food and Drug Administration (FDA) on May 20.
This medication is a tyrosine kinase inhibitor (TKI) that targets cancer cells, used in treating conditions such as chronic myeloid leukaemia (CML), Philadelphia chromosome-positive acute leukaemia, gastrointestinal stromal tumours (GIST), and rare skin cancers (DFSP).
Imcranib 100 treatment is now available at Chulabhorn Hospital, substantially reducing treatment costs and enhancing access to care for Thai patients, reported Bangkok Post.

“This groundbreaking achievement not only alleviates the burden of cancer for Thai citizens but also enhances Thailand’s capacity in pharmaceutical formulation, manufacturing, quality control, pharmacological testing, and regulatory processes,” the statement said.
“It establishes the groundwork for future cancer drug development and bolsters national drug security for sustainable public health improvement.”
In addition, the Chulabhorn Research Institute has successfully developed Thailand’s first targeted biological drug, Trastuzumab, registered as Herdara, which also received FDA approval on May 20.
Herdara, developed entirely by Thai researchers without foreign technology transfer, marks a significant milestone in achieving biopharmaceutical self-reliance.
Latest Thailand News
Follow The Thaiger on Google News:


























