Thailand’s first Covid-19 vaccine waits for approval from local FDA
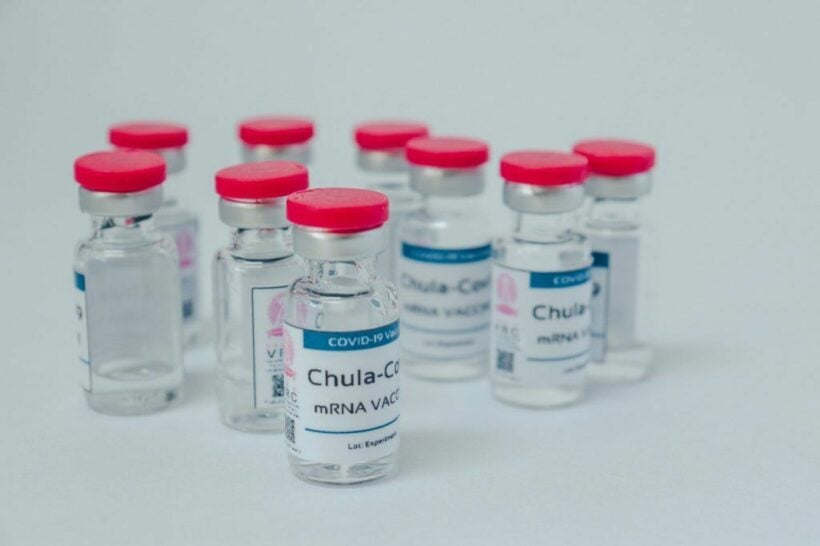
Better late than never. Thailand’s first Covid-19 vaccine, ChulaCov19, has shown impressive results in the first and second rounds of human trials. The director of the vaccine project claims the Thai vaccine is safe and more effective than Pfizer vaccines used in Thailand. The developing team expects the vaccine to be approved by Food and Drug Administration before the end of 2022.
ChulaCov19 is the first mRNA-based vaccine developed and produced in Thailand by the Chula Vaccine Research Centre at Chulalongkorn University and King Chulalongkorn Memorial Hospital in Bangkok. The director of the developing team, Kiat Rakroungtham, explained to Thai media that the trial results were impressive. He claims that the antibody generated by ChulaCov19 were higher than Pfizer vaccines used in Thailand.
He revealed that Thai vaccine manufacturer, BioNet-Asia had already produced the first batch of vaccines, and they all passed the quality testing phase. He updated that the developer had already sent the related document to the Food and Drug Administration and expect that the vaccine would be approved for distribution by the end of this year. The first two phases were done with volunteers, but the team will now launch the other two trials with select group of residents after receiving approval.
Kiat added that Thailand is now studying and developing 3 vaccines, including ChulaCov19 and Bai Ya vaccine by Chulalongkorn University and another vaccine by the Government Pharmaceutical Organisation.
He noted that Thailand has the in-house technology which allows the development, design, testing and production of Covid-19 vaccines within the country… “so we can handle whether it is a new variant or a new disease”.
“Vaccines will be developed faster, and Thailand can share them with neighboring countries.”
SOURCE: Khaosod
Latest Thailand News
Follow The Thaiger on Google News: