Developers of Thai mRNA vaccine call for it to be fast-tracked for emergency use
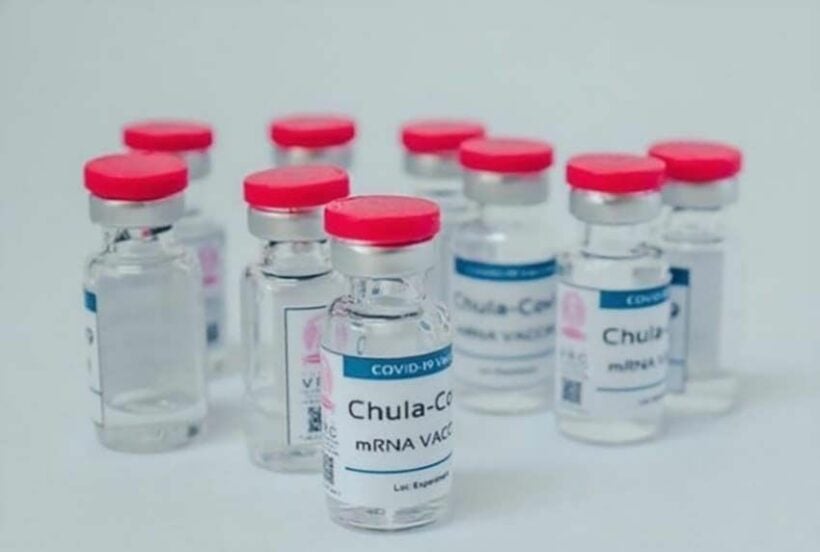
The developers of Thailand’s first mRNA-based Covid-19 vaccine are urging the Food and Drug Administration to approve it for emergency use during the booster roll-out. The Bangkok Post reports that, in a bid to become self-reliant in vaccine manufacture, the government has allocated a budget of 1 billion baht to developers.
The mRNA-based ChulaCov19 vaccine is being developed at Chulalongkorn University, under the leadership of Kiat Ruxrungtham. He says the team is now concerned that by the time the development process is complete, at least 70% of the population will already be fully vaccinated. He is calling on the FDA to allow the ChulaCov19 vaccine to be approved for emergency use in human trials, alongside the booster vaccines currently in use.
“We are going to consider the case with the FDA to reach a conclusion. If yes, we might see a place to use it for the booster vaccine.”
The developer of another Thai vaccine, the inactivated HXP-GPOVac, agrees, saying normal FDA regulations risk causing further delay in bringing local vaccines to market. Both HXP-GPOVac and ChulaCov19 are in phase 2 of human trials, with the tobacco-based vaccine from Baiya Phytopharm embarking on phase 1.
For more information on Covid-19 Insurance, CLICK HERE.
SOURCE: Bangkok Post
Latest Thailand News
Follow The Thaiger on Google News: