Experimental antiviral drug Remdesivir fails first clinical trial
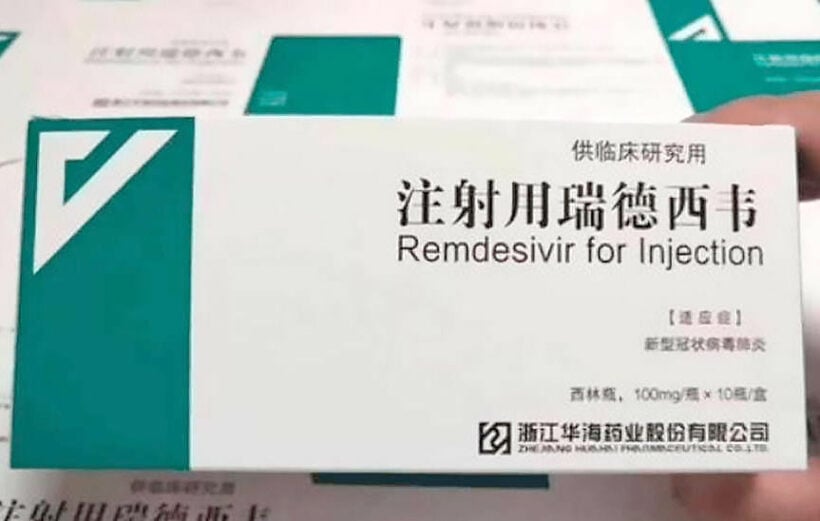
Reports are emerging that the experimental antiviral drug Remdesivir, developed by the American pharmaceutical company Gilead Sciences, has failed in its first randomised clinical trial to treat Covid-19, according to draft documents accidentally published by the World Health Organization and seen by the Financial Times. Gilead disputed how the now deleted post characterised the findings, saying the data showed a “potential benefit.”
Gilead originally developed Remdesivir as a treatment for the Ebola virus, where it showed promise at stopping the virus from replicating in clinical trials, but it was never approved.
Numerous media report that researchers in China carried out a study on 237 patients, giving the drug to 158 and comparing their progress with a control group of 79.
According to a summary Remdesivir was “not associated with a difference in time to clinical improvement” compared to the control group. Use of the drug was not associated with patients getting better faster, and 13.9% of patients receiving the drug died, versus 12.8% getting standard care.
A spokesman for Gilead told media, “We believe the post included inappropriate characterisations of the study,” saying it was terminated early due to low enrollment and was not statistically meaningful.
“As such, the study results are inconclusive, though trends in the data suggest a potential benefit for Remdesivir, particularly among patients treated early in disease.”
The clinical trial does not represent the final word on the matter and there are several large-scale trials in advanced stages that should soon provide a clearer picture.
Earlier this month, a study in the New England Journal of Medicine showed positive results for Remdesivir, with 68% of patients improving on the drug. But the study was not an official trial but rather the collation of data from patients who had been given the drug on a “compassionate use” basis, and and was not a double-blind study or compared to any control group. The scientists behind the study and even Gilead warned at the time that it was not conclusive.
Remdesivir, which is normally given intravenously, was among the first drugs proposed as a treatment for the virus and as such still has great hopes riding on it. It belongs to a class of drugs that act on the virus directly as opposed to controlling the abnormal and often lethal autoimmune response it causes. It mimics one of the four building blocks of RNA and DNA and gets absorbed into the virus’s genome, which in turn stops it from replicating.
But the drug is highly toxic and many medical experts were already warning against its use in the early days of the outbreak, as severe or critically infected patients died after using it.
Many doctors are warning that all clinical trials conducted influenced by major pharmaceutical entities should be regarded with extreme caution as they for-profit organisations.
SOURCES: Thailand Medical News | Financial Times
Latest Thailand News
Follow The Thaiger on Google News: