Chinese Covid-19 vaccine reported as just over 50% effective, down from 78%
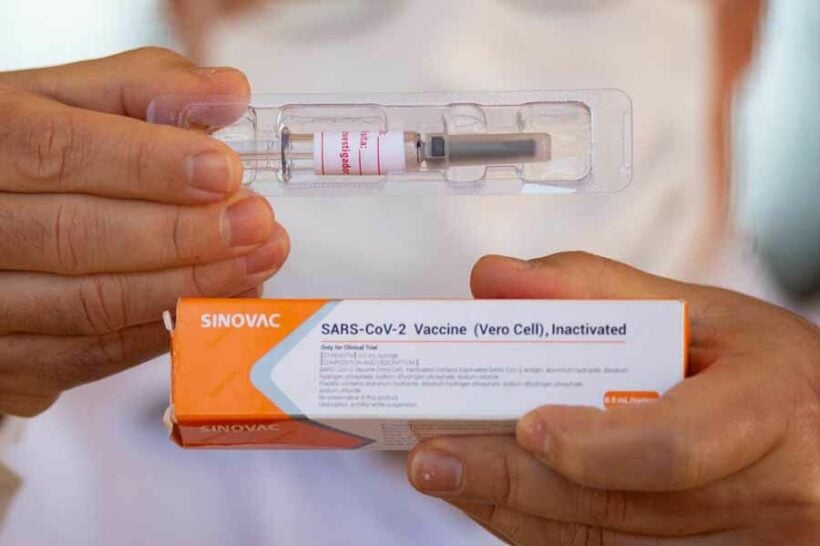
Confusion reigns over the exact efficacy rate of a Chinese Covid-19 vaccine due to be rolled out in Thailand next month. Thailand has ordered 2 million doses of the Sinovac Biotech vaccine, while its rollout among high-risk groups in Indonesia is expected to begin this week. However, questions now hang over the vaccine’s efficacy rate, originally reported as 78% in trials in Brazil but more recently downgraded to just over 50%. In total, 4 different efficacy rates have been reported, depending on the country conducting trials.
In Indonesia, where President Joko Widodo is set to get his first dose of the jab today, data from a local trial indicates an efficacy rate of 65%. However, with only 1,620 participants, the trial is considered too small for the data to have much value. Last month, Turkey reported an efficacy rate of 91.25% in its trial, which was also deemed too small to provide meaningful statistics.
The largest trial of the Chinese vaccine has been in Brazil, with 13,000 participants. There, however, 2 quite different efficacy rates have been reported. Just last week, the Butantan Institute, which partnered with Sinovac for the trials, reported that the vaccine was 78% effective in preventing mild cases of the virus and 100% effective in stopping severe and moderate infections.
However, the institute was forced to revise those figures yesterday, confirming instead an overall efficacy rate of 50.38%. The decreased rate comes after researchers came under pressure for a lack of transparency in reporting trial data. Ricardo Palacios from Butantan says the revised figure includes cases categorised as “very mild”, as no medical treatment was required.
It’s understood the Butantan Institute delayed announcing its results 3 times, which they say was due to a confidentiality clause in the contract with Sinovac. The disparity in reporting has raised some questions about the Chinese vaccine, with concerns that it is not receiving the same level of scrutiny as those produced in Europe or the US.
However, it’s not the first time there has been confusion over efficacy rates, with AstraZeneca initially reporting 2 different rates depending on the dosage administered. The Chinese vaccine still meets the efficacy threshold required for regulatory approval, at over 50%.
Latest Thailand News
Follow The Thaiger on Google News:


























