US approves emergency Covid antibody therapy, G20 nations push for vaccine availability

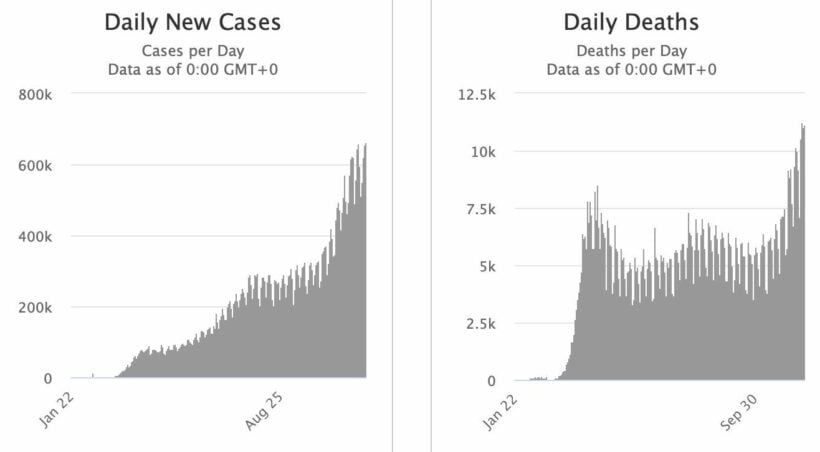
US drug regulators have approved an emergency Covid antibody therapy as cases surpass 12 million, keeping the country as the highest in the world for the level of new Covid cases. Meanwhile, G20 nations are pushing for vaccine availability as more areas in the world are experiencing closures due to spikes in the amount of virus cases.
The antibody therapy is the same that was used to treat US President Donald Trump after he tested positive for the virus.
Regeneron’s Regen-COV2 “antibody cocktail” is designed so that the two antibodies seek out and bind to the coronavirus’ spike protein to prevent it from entering healthy human cells.
Stephen Hahn, the commissioner of the US Food and Drug Administration says it was the second therapy that received approval on an emergency status.
“Authorising these monoclonal antibody therapies may help outpatients avoid hospitalization and alleviate the burden on our health care system.”
Regeneron says it expected to have doses ready for 80,000 patients by the end of this month with that number expected to rise to 300,000 available doses by the end of January 2021. The doses are available to patients at no out of pocket cost due to the terms of a US government programme. But the availability of the doses isn’t immediate, and many American travellers are rejecting warnings by packing airports over the Thanksgiving holiday.
Pfizer and its German partner BioNTech are also in the race for treatment approval as it has become the first in the US or Europe to request emergency approval based upon its trials showing its version of the vaccine is 95% effective. Moderna is also reporting that its vaccine is 95% effective, but that hasn’t stopped concerns over whether there will be sufficient access to the vaccines worldwide.
SOURCE: Bangkok Post
Latest Thailand News
Follow The Thaiger on Google News:


























