Government ramps up production of 5 anti-Covid-19 medicines, procurement of others
The Government Pharmaceutical Organisation is increasing production of five drugs used treat Covid-19 to ensure adequate stocks for all patients.
A GPO spokesmansaid that excluding Favipiravir also known as Avigan, Thailand has five drugs to treat Covid-19, totalling 39.6 million tablets: there are 1.8 million tablets of Chloroquine, an anti-malarial drug; 30.6 million tablets of an antiretroviral drug containing Lopinavir and Ritonavir, used to treat HIV/AIDS; 1.9 million tablets of the antiretroviral drug Darunavir; 1.9 million tablets of Ritonavir and 3.4 million tablets of Azithromycin, an antibiotic used to treat bacterial infections.
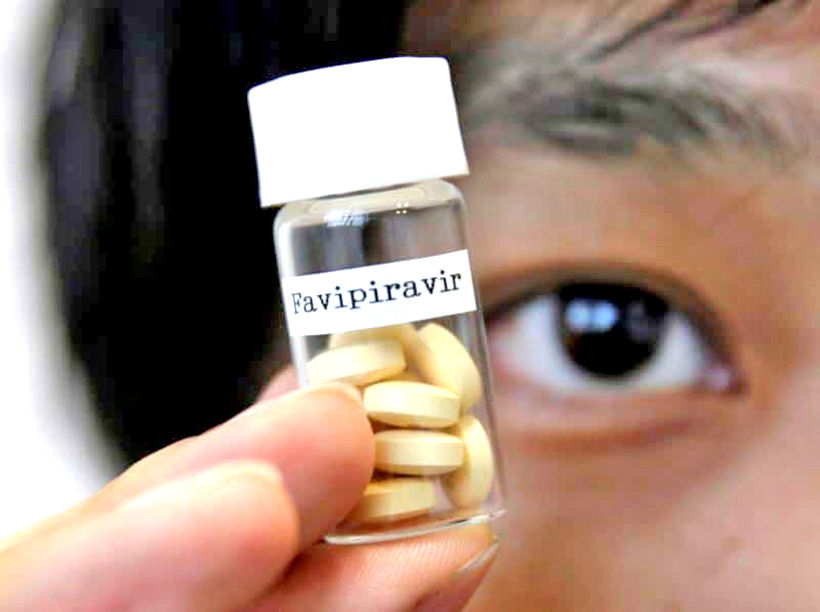
The spokesman says the GPO has expedited the purchase of antiviral drugs — Favipiravir and Hydroxychloroquine.
“We ordered Favipiravir from Japan and China of which 187,000 tablets have been delivered, while another 103,860 tablets will be delivered by the end of April. Meanwhile, we have already ordered 1.09 million tablets of Hydroxychloroquine from domestic manufacturers.”
SOURCE: The Nation Thailand
Join the conversation and have your say on Thailand news published on The Thaiger.
Thaiger Talk is our new Thaiger Community where you can join the discussion on everything happening in Thailand right now.
Please note that articles are not posted to the forum instantly and can take up to 20 min before being visible. Click for more information and the Thaiger Talk Guidelines.
Leave a Reply
You must be logged in to post a comment.